Looking Good Tips About How To Draw D Orbitals

How to draw shapes of d orbitals content link disclosure.
How to draw d orbitals. Draw shapes of 2s and 2p orbitals. Point to an atom where the node of the orbital is to be attached. To draw the orbital diagram for an atom, follow these basic steps.
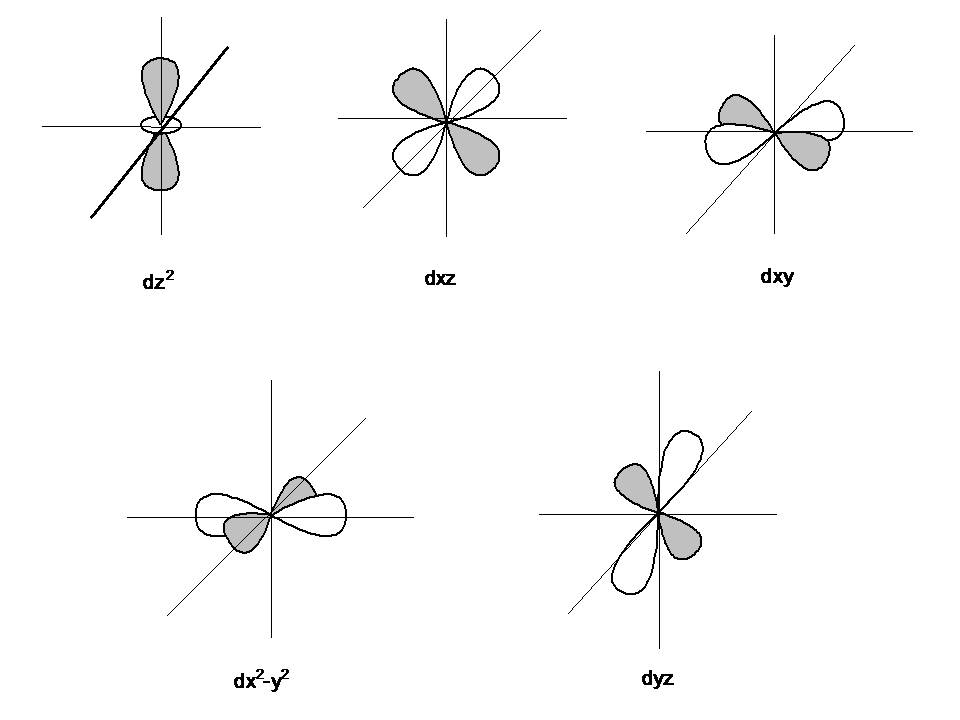
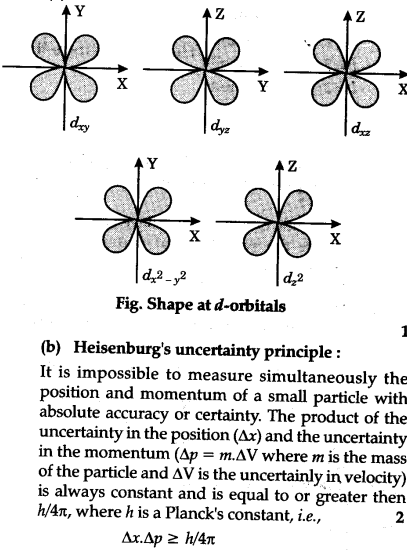
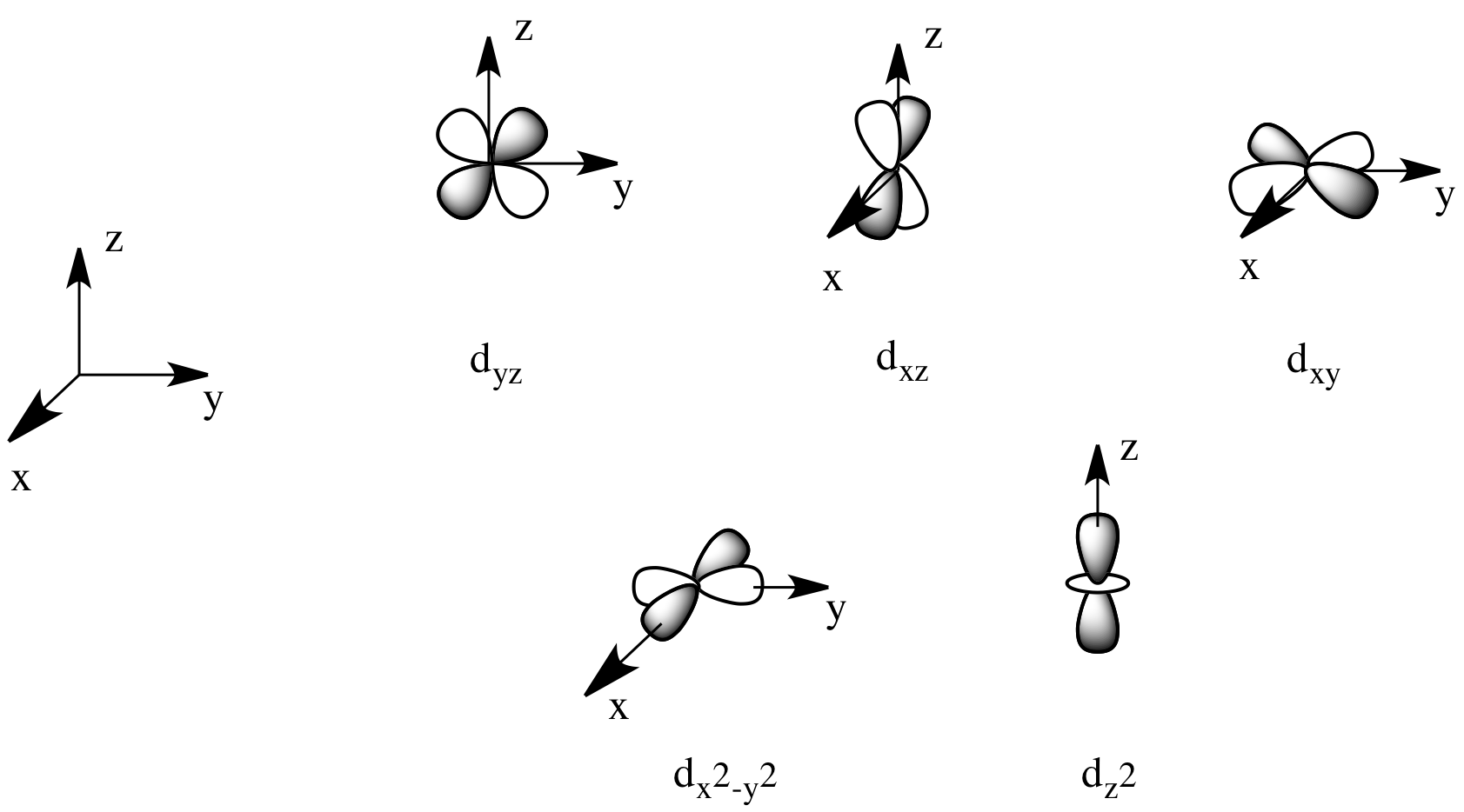
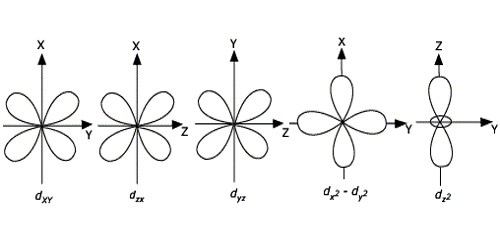
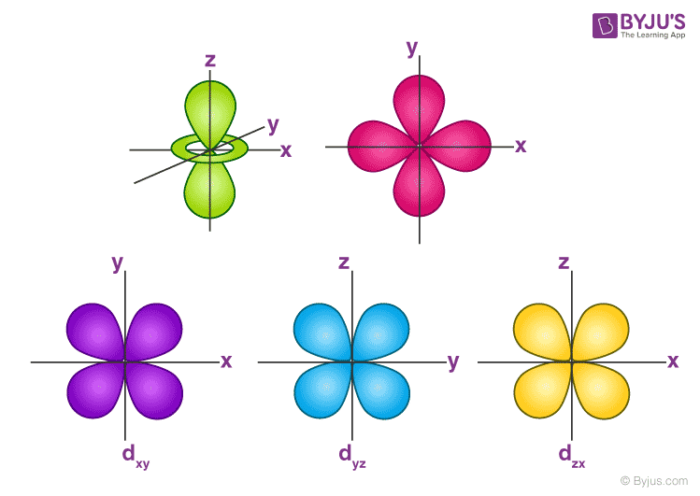
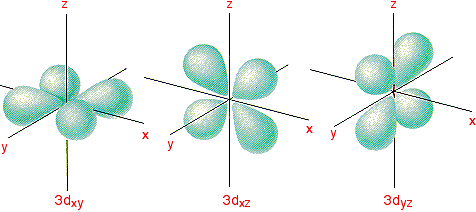
Draw the shapes of the 1s, 2s, 2 p x , 2 p y , 2 p y , 3 d x y , 3 d x z , 3 d y x , 3 d x 2 − y 2 a n d 3 d z 2 orbitals. P orbital has 3 orientations. The energy of all five d orbitals is the same.
It implies that d subshell has 5 orbitals i.e. Five electron cloud and can be represent as follows: Write the electron configuration for an atom to determine which orbitals should be.
Draw the shapes of various `p` and `d` orbitals. 1215 draw the shape of an s orbital and the shapes of the p x p y and p z orbitals. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.
They have an even more complex angular distribution than. Find the number of electrons in an atom.